Health and Medicine
92 CRISPR and Gene Editing Technology
Kylie Towery; Andrew Scott; and Emma Volding
Introduction
Gene editing is a group of technologies that give a scientist the ability to alter an organism’s existing genome. This revolutionary approach allows for precise manipulation of genes and holds immense clinical and therapeutic potential. One of the most prominent technologies in gene editing is CRISPR, which stands for a Clustered Regularly Interspaced Short Palindromic Repeats. Originally discovered through studies of adaptive immune responses in bacterial models, CRISPR is a type of genome editing naturally occurring in these types of bacteria. Today, scientists are utilizing this template to modify and edit strands of DNA in living organisms. CRISPR represents a revolutionary advancement in gene editing, with applications that include treating a wide variety of clinical and therapeutic applications, such as blindness, cancer, and even the rapid development of disease testing like for Covid-19 (Science & Tech Spotlight: CRISPR Gene Editing, 2020).
Connection To STS
Gene editing technology, while scientifically groundbreaking, is also deeply connected and tied to Science, Technology, and Society (STS) studies due to its overarching societal implication. Gene editing enables precise modifications of genetic material, but also raises significant societal and ethical considerations. CRISPR-cas9 houses potential to revolutionize medicine, agriculture, and biotechnology, offering solutions to genetic disorders and enhancing crop resilience. However, these benefits come with challenges, like unintended genetic mutations and ethical dilemmas surrounding germline editing (Janik et. al 2020). For example, while it enables the treatment of genetic disorders and enhances crop resilience, it also raises concerns about unintended mutations and germline modifications along with their long-term societal impacts. These concerns have intensified with recent uses of CRISPR in human embryos, sparking debates about the long-term consequences of editing the human genome. There is a need for robust regulation and ethical guidelines to navigate the consequences that may come with this. Gene editing technologies, like CRISPR, are not just scientific tools–they reflect and shape societal norms, values and power structures. The development and application of these technologies are influenced by cultural, political, and economic factors, which in turn affect public perception and policy-making. Socially, the technology could exacerbate existing inequalities if access to gene editing is limited to those with significant resources. Access to CRISPR might grow into a societal justice issue if only the wealthy can afford genetic enhancements, potentially creating a new class of genetically privileged individuals (Sargent et al., 2017). CRISPR-cas9 and other gene editing technologies have the potential to revolutionize disease therapy and drastically improve patient outcomes, but many ethical considerations must be taken in order to ensure safety and efficacy. Therefore, public policy, ethics, and regulatory frameworks must evolve alongside these technologies to ensure equitable and safe implementation.
Figure 1: Gene Editing depicted as Enhancing Features such as Intelligence or Eye Color
Understanding CRISPR Technology
In 1987, CRISPR Technology was discovered in the immune system of archaeal genomes as a form of natural adaptation to defending microbes from bacteriophage infection (Ishino 2018). By 1993, further repeats were found in Haloferax mediterranei, and later research in the 2000s confirmed that the CRISPR-Cas (CRISPR-associated) system provides immunity against bacteriophages through spacer sequence observations. It is believed that CRISPR models applied to humans and other organisms can be used to target viral infections.
At the heart of this system lies CRISPR-Cas9, which functions through interaction between the Cas9 enzyme and a strand of guide RNA (gRNA). The molecular guide, gRNA, directs the Cas-9 enzyme towards the target molecular strand of DNA. Once at the target location on the DNA strand, the enzyme essentially acts as a pair of molecular scissors and cuts the desired strand. This precise targeting allows scientists and researchers to effectively delete, insert, or modify a specific strand of DNA, virtually acting as a natural DNA editing tool.

Figure 2: CRISPR depicted as the “molecular scissors for gene editing”
Applications in Disease Therapy
CRISPR offers the transformative potential to treat a wide range of diseases, from rare genetic conditions to infectious diseases and cancer. Utilizing the defense mechanism of observed bacterial immune systems, CRISPR can create new pathways of engineered microorganisms for disease therapy. It allows for permanent modification of a genomic target sequence that could potentially repair DNA damage (Rodriguez-Rodriguez 2019). The creation of such can be manipulated to induce certain responses in programmed mutation. CRISPR can have applications in metabolic engineering, disease therapy, agriculture, and biomedical sciences. With the recent Covid-19 outbreak, CRISPR can be applied to Covid testing and treatment. Much of CRISPR’s popularity comes from its high effectiveness and success rates. It is a form of genome editing that does not cause lethality to any organism.
In humans, gene editing technologies, allow for precise modifications to DNA, which can be used to correct genetic mutations responsible for various diseases. Some of these diseases include sickle cell anemia, cystic fibrosis, and hemophilia. Cystic Fibrosis is a genetic disorder caused by mutations in the CFTR gene, that causes the production of thick mucus that can clog the lungs and obstruct the pancreas. CRISPR technology has shown the ability to correct these mutations by targeting and repairing the defective CFTR gene. Research has been done with this disease and CRISPR that would allow for a one time, permanent solution (Maeder & Gersbach, 2016; U.S. National Library of Medicine, n.d). Hemophilia is a bleeding disorder caused by mutations in the genes responsible for blood clotting factors. CRISPR has been used to target and correct these mutations, specifically in hemophilia B, which is caused by a deficiency in factor IX. Gene editing can potentially provide a long-term solution by permanently correcting the genetic defect, which then reduces or eliminates the need for regular clotting factor infusions (Maeder & Gersbach, 2016; U.S. National Library of Medicine, n.d).Sickle Cell Disease is a condition that is characterized by the production of abnormal hemoglobin, leading to the formation of rigid, sickle-shaped red blood cells that can block blood flow and cause severe pain and organ damage. CRISPR has been used to edit the genes in hematopoietic stem cells to produce normal hemoglobin, offering a potential cure for this debilitating disease. Clinical trials that have been conducted have shown promising results, with patients experiencing significant improvements in symptoms and quality of life (Maeder & Gersbach, 2016; U.S. National Library of Medicine, n.d). Advancements, such as these three, highlight the potential of CRISPR and other gene-editing technologies in treating genetic disorders that were previously considered incurable by medical professionals.
Gene editing technologies, like CRISPR, are headed for some further clinical trials. These trials will consist of ex vivo experimental testing, a way of testing a patient’s cells outside of the patient’s body. This is opposite of in vivo testing, which takes place within the patient. This will allow a safer form of testing that will not result in any unintended consequences like off-target editing. It ensures that the gRNA will guide the enzyme to splice the correct location on the DNA strand before reintroduction into the patient’s body (Hirakawa 2020). Further translation from lab bench experiments into a more clinical setting is needed to fully realize CRISPR as a substantial form of disease therapy.
Ethical Considerations and Challenges
With CRISPR being at the forefront of genome editing, it is no surprise that it raises ethical questions since it is an advanced form of biotechnology. Gene editing can have consequences such as unintentional changes in the genome. Other issues that groups point to are “designer babies” with enhanced genetic traits as potential unethical outcomes of this technology. Likewise, the ethical considerations extend to the potential for CRISPR to be used for non-therapeutic enhancements, such as selecting for favorable traits like higher intelligence or physical appearance (de Lecuona et al., 2017). There are also environmental concerns as gene editing could disrupt the natural selective pathway all organisms have taken to survive. Worldwide legislation must be unanimously agreed upon regarding the growth of gene editing technology in order for it to be used for only the betterment of the human race (Ayanoglu 2020). Additionally, the use of CRISPR in human embryos raises profound questions about the moral status of embryos and the implications of germline modifications, which are changes that can be passed on to future generations. This has led to debates about the long-term impact on the human gene pool. The American Medical Association’s Code of Ethics highlights that germline manipulation could result in “unpredictable and irreversible results that adversely affect the welfare of subsequent generations” (Sargent, Johnson, & Sarata, 2017).
CRISPR Technology has several limitations that may prevent its immediate commercialization. These limitations include immunotoxicity, DNA Damage/Apoptosis, or off -target effects. A common problem with CRISPR technology is specifically the off-target effect, which is when the Cas-9 enzyme cleaves a different site on the DNA strand than intended. After sufficient investigation, researchers suggest that the amount of gRNA and Cas-9 must be optimized and proportionate, and must be completed through direct delivery (Joshi 2024). This will ensure that the system is not thrown out of balance and will only target the intended sequence. The ethical considerations and technological challenges present many hurdles for CRISPR to overcome in order for it to be fully realized in a clinical setting.
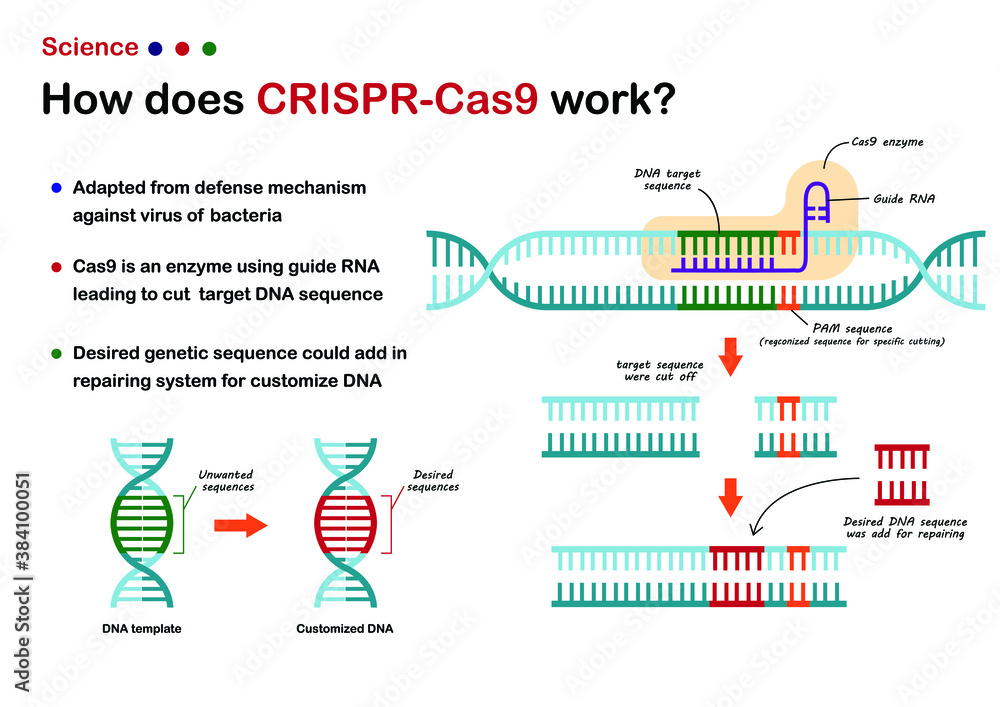
Figure 3: Visual Depicting Mechanism of CRISPR-Cas9 System
THE DEBATE ON CRISPR
CRISPR-Cas9 gene editing offers notable advantages, including precise gene modification, potential treatments for genetic disorders like sickle cell anemia, advancements in cancer therapies, and the development of disease-resistant crops and animals. It also accelerates functional genomics research and has applications in environmental conservation.However, challenges accompany these benefits, such as ethical concerns regarding germline editing, unintended off-target effects, regulatory hurdles, and the potential for unforeseen ecological impacts. Balancing these pros and cons is crucial as CRISPR technology continues to evolve.
Conclusion
CRISPR gene editing is a powerful yet still-evolving technology that holds potential to revolutionize personalized medicine and treat previously incurable conditions by offering unprecedented precision and efficiency in targeting specific genes. However, numerous research studies at this point can have conflicting evidence regarding its safety and efficacy. Its use must be governed by caution and ethics particularly when applied to human embryos or germline cells. While current research shows promise, much remains unknown, and today’s conclusions may evolve with time. Moving forward, the scientific community, ethicists, and policymakers must work together to ensure that CRISPR is used responsibly, equitably, and safely. With continued dedication and ethical vigilance, CRISPR technology has the ability to usher in a new era of personalized medicine, where genetic disorders or diseases are no longer a barrier to health and well-being.
Chapter Questions
- SHORT ANSWER: How does gene editing allow scientists to alter an organisms genome?
- TRUE OR FALSE: gRNA stands for guide-RNA.
- SHORT ANSWER: What does CRISPR stand for?
References
Ayanoglu, F. B., Elcin, A. E., & Elcin, Y. M. (2020). Bioethical Issues in Genome Editing by the CRISPR/Cas9 Technology. Turkish Journal of Biology, 44(2), 110–120. https://doi.org/10.3906/biy-1912-52
de Lecuona, I., Casado, M., Marfany, G., Lopez Baroni, M., & Escarrabill, M. (2017a, December 19). Gene editing in humans: Towards a global and Inclusive Debate for Responsible Research. The Yale journal of biology and medicine. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5733855/
Hirakawa, Matthew P., Krishnakumar, R., Timlin, Jerilyn A., Carney, James P., & Butler, Kimberly S. (2020). Gene editing and CRISPR in the clinic: current and future perspectives. Bioscience Reports, 40(4). https://doi.org/10.1042/BSR20200127
Ishino, Y., Krupovic, M., & Forterre, P. (2018). History of CRISPR-Cas from Encounter with a Mysterious Repeated Sequence to Genome Editing Technology. Journal of Bacteriology, 200(7), e00580-17. https://doi.org/10.1128/JB.00580-17
Janik, E., Niemcewicz, M., Ceremuga, M., Krzowski, L., Saluk-Bijak, J., & Bijak, M. (2020). Various Aspects of a Gene Editing System—CRISPR–Cas9. International Journal of Molecular Sciences, 21(24). https://doi.org/10.3390/ijms21249604
Joshi, S., Verma, D., & Gupta, R. K. (2023). CRISPR-Cas System in Translational Biotechnology. Elsevier Shop. https://shop.elsevier.com/books/crispr-cas-system-in-translational-biotechnology/joshi/978-0-323-91808-4
Maeder, M. L., & Gersbach, C. A. (2016). Genome-editing Technologies for Gene and Cell Therapy. Molecular Therapy, 24(3), 430–446. https://doi.org/10.1038/mt.2016.10
Rodriguez‑Rodriguez, D., Ramirez‑Solis, R., Garza‑Elizondo, M., Garza‑Rodriguez, M., & Barrera‑Saldaia, H. (2019). Genome editing: A perspective on the application of CRISPR/Cas9 to study human diseases (Review). International Journal of Molecular Medicine, 43(4), 1559–1574. https://doi.org/10.3892/ijmm.2019.4112
Sargent, J. F., Johnson, J. A., & S, A. K. (2017, September 12). CRISPR gene editing research in embryos generates … Congregational Research Service. https://crsreports.congress.gov/product/pdf/IN/IN10773/9
U.S. Government Accountability Office. (2020). Science & Tech Spotlight: CRISPR Gene Editing. Www.gao.gov, GAO-20-478SP. https://www.gao.gov/products/GAO-20-478SP
U.S. National Library of Medicine. (n.d.-a). What are genome editing and CRISPR-Cas9?: Medlineplus Genetics. MedlinePlus. https://medlineplus.gov/genetics/understanding/genomicresearch/genomeediting/
Schmerker, J. (2024, April 15). CRISPR-Cas9: 10 pros and 7 cons. Integrated DNA Technologies. https://www.idtdna.com/pages/community/blog/post/crispr-cas9-what-are-the-10-pros-and-7-cons
Image Sources
“Science illustration show CRISPR – Cas 9 work for cut and edit DNA genetic sequence as novel technique of molecular engineering.” by trinset on Adobe Stock.
“Illustration depicting CRISPR molecular scissors for gene editing.” by TopMicrobialStock on Adobe Stock.
“CRISPR CAS-9 – Genetic Engineering – Stock Illustration.” by Panuwach on iStock
AI Acknowledgement
ChatGPT was used by Andrew for the Conclusion Section. The following prompt was used:
Write a conclusion paragraph to an essay about CRISPR technology using these guidelines:
– Summarize the key points discussed, emphasizing the potential of CRISPR technology in gene editing and disease therapy.
– Emphasize the importance of ongoing research and ethical considerations in harnessing the full potential of CRISPR.
– Conclude with a hopeful outlook for the future of CRISPR technology and its potential to transform healthcare and improve patient outcomes.
Copilot was used to to generate ideas for the Connection to STS portion of the chapter. Kylie sent all of her sources to Copilot and asked for a paragraph relating STS to gene editing technology. It sent an abundance of sentences. Some of which were completely discarded, while others were edited or rearranged prior to inserting them into the chapter.
Copilot was used for a portion of the first paragraph of the Ethical Considerations and challenges portion. This prompt was used :Write a few sentences relating gene editing technology to STS using information from this source, include APA in text citations Sargent , J. F., Johnson , J. A., & Sarata, A. K. (2017, September 12). CRISPR Gene Editing Research in Embryos Generates Scientific and Ethics Debate. Congress.go. https://crsreports.congress.gov/product/pdf/IN/IN10773/9. Copilot included several sentences about the ethics of gene editing, which were then altered, added onto, and rearranged prior to their use within this chapter.
ChatGPT was used by Emma to include “The Debate on CRISPR” section in this chapter in order to ensure the phrasing aligned with the original work by the authors.

